We’re dreaming of a scientific Christmas, and nothing screams holiday chemistry more than making your own pH paper from a Poinsettia. Yeah, we’re excited, so put on those ugly Christmas sweaters and get in the holiday spirit!
The Chemistry of a Poinsettia
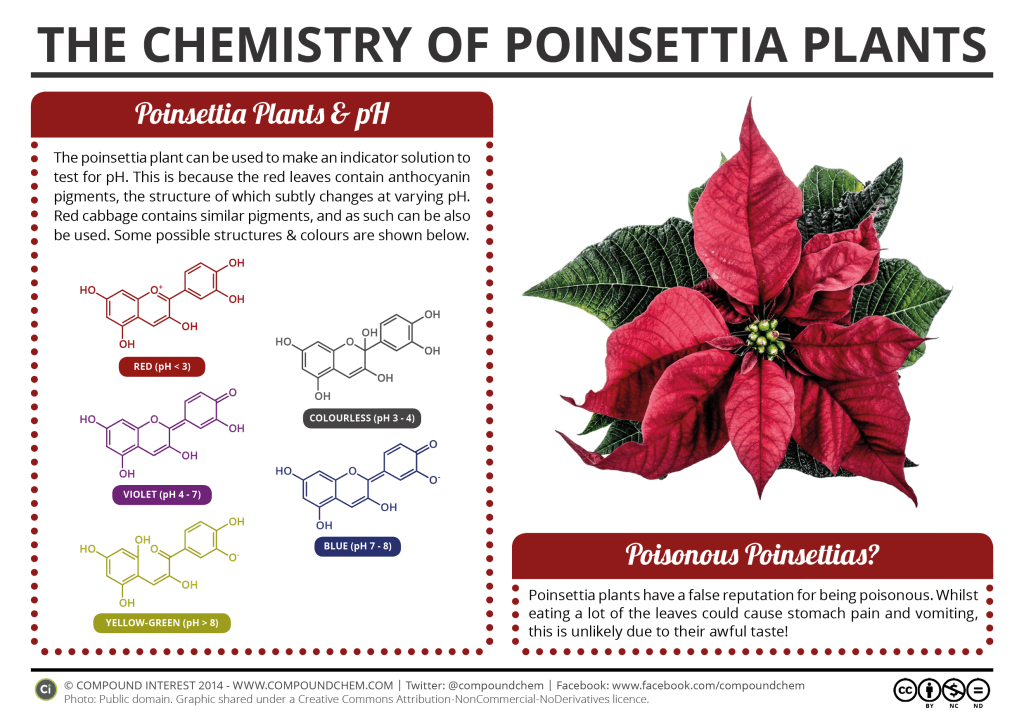
Let’s start by looking at how this little science project is possible. Poinsettia plants can be used to make an indicator solution to test for pH because the red leaves contain anthocyanin pigments. The structure of these anthocyanin pigments change slightly at varying pH levels, similar to red cabbage, which we did here.
Remember, pH is a measure of how acidic or alkaline a solution is. For a more in-depth description, read our article about acids, bases and the pH scale. To sum it up, the pH scale measures from 0-14. The lower the pH, the more acidic the solution. The higher the pH, the more alkaline the solution.
There are a number of pH indicators that can be used to determine the pH of a solution, but we can also use a poinsettia plant to make a pH indicator. As we mentioned earlier, the plant’s leaves contain pigments called anthocyanins, which are responsible for the red coloration in Poinsettias, as well as cabbage, blueberries and raspberries.
At a pH of 3 or below, anthocyanin is orange or red. As you climb the pH scale, they tend to appear more colorless just below a neutral pH of 7. Then, as you continue to increase the pH, they begin to give off a green, blue or purple color. Thus, you can use the poinsettia to make pH paper that can determine whether a solution is acidic or basic. Let’s get started!
Making Poinsettia pH Paper
You will need the following materials:
- Poinsettia ‘flowers’ (red leaves)
- Beaker or cup
- Hot plate or boiling water
- Strainer
- Scissors or a blender
- Filter paper or coffee filters
- Vinegar, Lemon juice or HCl (hydrochloric acid)
- Baking soda solution (2 g / 200 mL water), NaOH (sodium hydroxide) or KOH (potassium hydroxide)
Find as much materials as you can around the house. This should be a fun, easy experiment!
Procedure:
- Cut flower petals into strips or chop them in a blender. Place the cut pieces into a beaker or cup.
- Add just enough water to cover the plant material. Simmer until the color is removed from the plant, up to 10 minutes. Or, add already boiling water to the leaves. We recommend microwaving the chopped leaves with a bit of water for about a minute, then allow the mixture to steep, like a tea.
- Strain the liquid into another container, such as a petri dish or shallow, flat bowl. Discard the plant matter and filter.
- Saturate the clean filter paper or coffee filter with the poinsettia solution. Allow the filter paper to dry. You can then cut the colored paper with scissors to make pH test strips.
- To see if your pH paper works, let’s try dipping it into acidic and alkaline solutions.
- Use a dropper or a toothpick to apply a little liquid to a test strip. The color range for acids and bases will depend on the particular plant. If you like, you can construct a chart of pH and colors using liquids with a known pH so that you can then test unknowns. Examples of acids include hydrochloric acid (HCl), vinegar, and lemon juice. Examples of bases include sodium or potassium hydroxide (NaOH or KOH) and baking soda solution.
- Another way to use your pH paper is as a color-change paper. You can draw on pH paper using a toothpick or cotton swab that has been dipped in an acid or a base. Try drawing a holiday greeting card!
This science experiment was brought to you by Chemistry.About.com. Happy Holidays everyone!







Leave A Comment