If you are experiencing different results (taste of bitterness, then no taste at all) with the PTC paper, there might be a few explanations.
Sensitivity to PTC is based on a genetic disposition, so it seems likely that a positive one time should give a positive the next time. There does appear to be evidence that results with the same individual can vary as much as 8 times. This may be what you are experiencing.
It does make sense to limit intake of food or drink prior to the test, since it may be possible to offset or mask the effect. Have a supply of water available to rinse out your mouth (spit) to eliminate the bitter taste. There is also mention in some literature that smoking can diminish a person’s ability to detect PTC.
PTC paper is very stable, so it’s unlikely the paper is too old to work. A couple of other unlikely possibilities might be that the paper didn’t get proper soaking treatment during manufacturing or the paper was Control paper and not PTC. These situations are both very unlikely, and could be tested by trying a strip from another vial or batch.
The Glucose test strips were originally developed to test for glucose levels in urine for educational purposes (not medical diagnosis). Other uses include osmosis experiments where a glucose/starch solution is used to demonstrate the concept in a classroom setting.
The strips will also detect glucose levels in food, however, there are several things to consider:
1) The enzyme used to detect the glucose is specific for glucose sugar. Other sugars will not be detected by the test strip.
2) While exclusive for glucose sugar, other chemicals can interfere. The best (or worst) example is Vitamin C. The strips will not work well with foods high in Vitamin C.
3) In order for the enzyme to work properly, the solution being tested may need to sit for up to 3 hours to allow mutarotation to occur. (In most natural settings, the mutarotation has already occurred.)
Yes, we call it our Salinity Test Strip. This test strip measures the level of chloride present and can be used as an indirect indicator of sodium when the sodium is present due to sodium chloride salt.
If the sodium is due to NaCl and not from other sources, a ppm level of 500 ppm Cl would be equivalent to a ppm level of about 320ppm sodium based on the differences in molecular weight. A 1000ppm chloride result would therefore be about 640ppm sodium.
Procedure:
1. Nutrient broth medium inoculated with Escherichia coli and Staphylococcus aureus and incubated at 37C for 24 hours, “starting cultures”.
2. A standard plate count procedure performed using 24 hour cultures of Escherichia coli and Staphylococcus aureus. Cultures diluted (10-1, 10-2…10-8) in 0.75% saline solution, plated on nutrient agar medium and incubated at 37C for 24 hours. The number of colony forming units (CFU’s) determined and the starting culture bacterial concentration
determined.
3. In conjunction with the dilution procedure, 0.1 ml of each diluted bacteria suspension was then applied to a standard microscope slide and allowed to air dry. A Protein test strip pressed onto the air-dried bacteria suspension was then applied to determine the presence of protein.
Results:
The Protein test strips were able to detect 1.0 x 10^6 bacteria per ml.
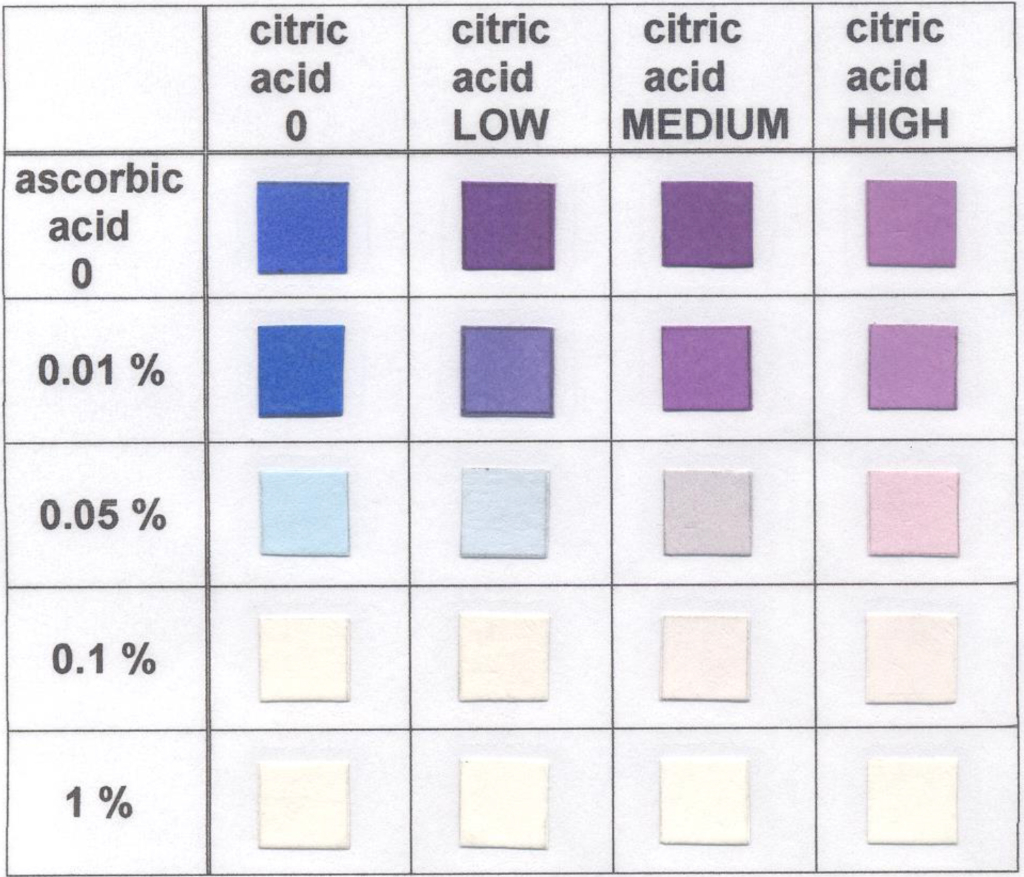
The Vitamin C test strip uses 2,6-dichlorphenolindophenol as a red-ox indicator. This indicator changes from blue to colorless as the amount of ascorbic acid (Vitamin C) increases. Unfortunately, this indicator also detects other acids by changing from blue to red.
To help offset this effect, we include buffer salts in the formulation. This works for many applications, but it doesn’t work as well when the other acid is present in large amounts (such as in some fruit). In this situation, the starting blue color becomes more lavender.
This is why we have constructed a color scheme showing the effect of citric acid on the Vitamin C strip. Citric acid was chosen because of the likelihood that it would be present in some of the same solutions being tested for Vitamin C. The mechanism for the color change is based on the effect of pH on the indicator.
Glucose Apart from glucose, no other compound in urine is known to give a positive reaction. False positive reactions can be produced by a residual of oxidative compounds, from cleansing agents, for example. Larger amounts of vitamin C (e.g. from tablets, antibiotics or fruit juices) can result in lower or false negative results.
Ketones Urine ketone bodies include acetoacetic acid, acetone and beta-hydroxybutyric acid, and they are produced exclusively in the liver. Ketones in the urine signalize an abnormal carbohydrate metabolism.
Beta-hydroxybutyric acid is not detected, as it is not a ketone. Phenylketones in higher concentrations interfere with the test by producing variable colors. Phthalein compounds interfere by forming a red color.
Protein The Protein test pad contains changes color in the presence of albumin. Other urine proteins are indicated with less sensitivity (e.g. globulins, mukoproteins, hemoglobin, Bence-Jones protein).
The protein test is not influenced by the urine physiological range of pH values, but in strongly alkaline urine (pH >8) or in urines with extremely high buffering capacity, the test can provide false positive results. In addition, the presence of polyvinylpyrrolidone (blood substitute), quinine or the disinfectants residue (quat-based) can lead to false positive results. The residues of disinfectants on the base of nonionic or anionic detergents can also cause false negative results.
pH The pH value of fresh urine from healthy individuals varies from a pH of 5 and 6 to a pH of 8, depending on the individual’s food intake. Prevalence of meat products in the diet lead to a more acidic pH level, while a lacto-vegetable diet causes more alkaline urine with a pH greater than 7. Any inorganic acidic or alkaline substances presented in urine can interfere with the test.
The SDS for each taste test paper lists the ingredients. The concentration of ingredients is usually so small, it is less than what would be considered hazardous. Aside from these small quantities, the cellulose paper is the only other ingredient. In addition, the proper use of each taste test is to touch the strip to the tongue. These taste strips have been used safely in classrooms for decades.
The manufacturing facility where the PTC paper is produced (Cottonwood, AZ) is a typical manufacturing site. We don’t manufacture nuts or other products usually associated with allergy concerns. Please note, however, that the facility is not certified or considered an allergy-friendly manufacturing facility. In addition, the raw materials used in producing the taste test papers are not procured in any special fashion. We have no assurance that they were produced in an allergy-free environment.
Despite the above information, if you have a concern about a possible allergic reaction, perhaps it would be best to abstain from the activity.
YES. In the case of the genetics taste test strips, both PTC and Sodium Benzoate are salts of benzoic acid. Any possible toxicity would be in grams per kilogram of body weight, which is millions of times greater than anything which would be found in our taste test strips. Phenylthiocarbamide (PTC) is present at only 20 micrograms per strip. At this level, the compound is negligible and harmless.
The taste test strips exhibit a taste due to a dominant allele on chromosome number seven, and the ability to taste these compounds is present in about 70% of the U.S. population. The ability to taste is due to two different sets of alleles. These compounds are present in various naturally occurring foods, and are selected due to their similarity to bitter alkaloids and cardiac glycosides, used by the plant to reduce browsing by herbivores.
Thus, their presence is a result of natural selection both for the plants which produce them and the animal which benefits from the ability to sense them. It is a benefit to be able to detect them and avoid bitter tasting foods, some of which might be harmful if swallowed. Hence, it is a trait selected for in populations evolving in an area which had/has such plants.
Unlike PTC, which taste bitter if an individual can taste it at all, Sodium Benzoate might taste sweet, salty, or bitter. It would generally taste salty to an individual who can taste the bitterness of PTC.
These test strips are selective for glucose. You will not get a reaction with soft drinks or regular sugar, as they primarily contain fructose or sucrose. In solutions containing oxidizers (for example, iodine solutions in diffusion/osmosis experiments), a false blank may be observed.
The Ascorbic Acid test strip is based on discoloration of blue red-ox indicator, depending on the concentration of ascorbic acid. The Ascorbic Acid test strip is calibrated from 0.01% (10 mg/100 ml) to 0.1% (100 mg/100 ml). Red-ox indicator is also sensitive to acidic compounds, and its blue color turns to purple in the presence of acids.
The Vitamin C strips are buffered to a certain extent to keep the blue color of the pad, but in the presence of strong acids, contained in some fruits or juices, the color can turn to a purple-pink instead of a blue-white. In such cases, we recommend using a special color chart (See “Ascorbic Acid Color Changes” chart below) which shows results for Vitamin C in the presence of different concentrations of citric acid. Lemons and limes contain the highest amount of citric acid at about 5 percent. Grapefruits and oranges also have high content of citric acid at about 2.5 and 2 percent, respectively.
In the “Ascorbic Acid Color Changes” chart (click button below), the values are as follows:
Low = 0.5%; Medium = 1%; High = 5%